New applications of AI in clinical trials
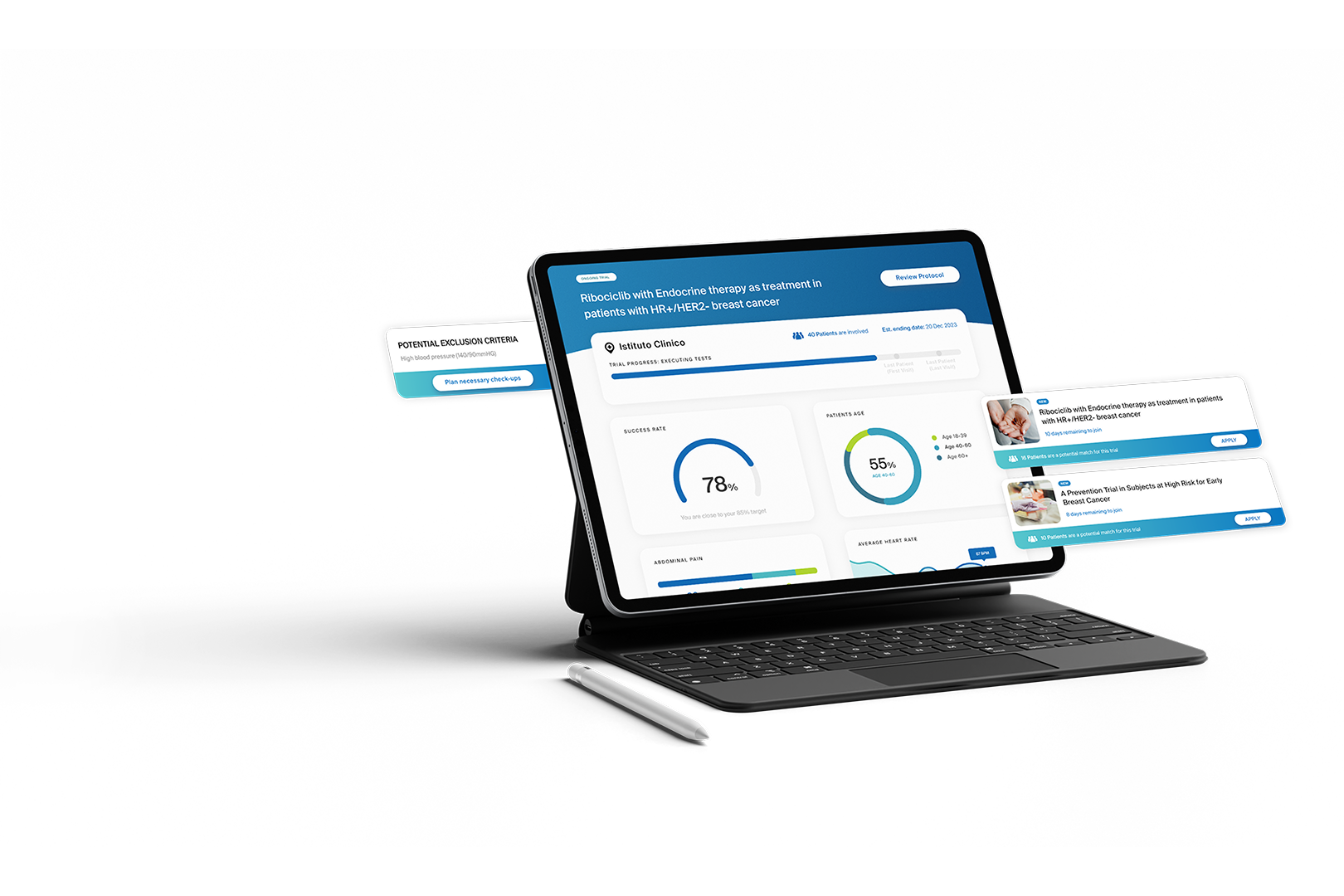
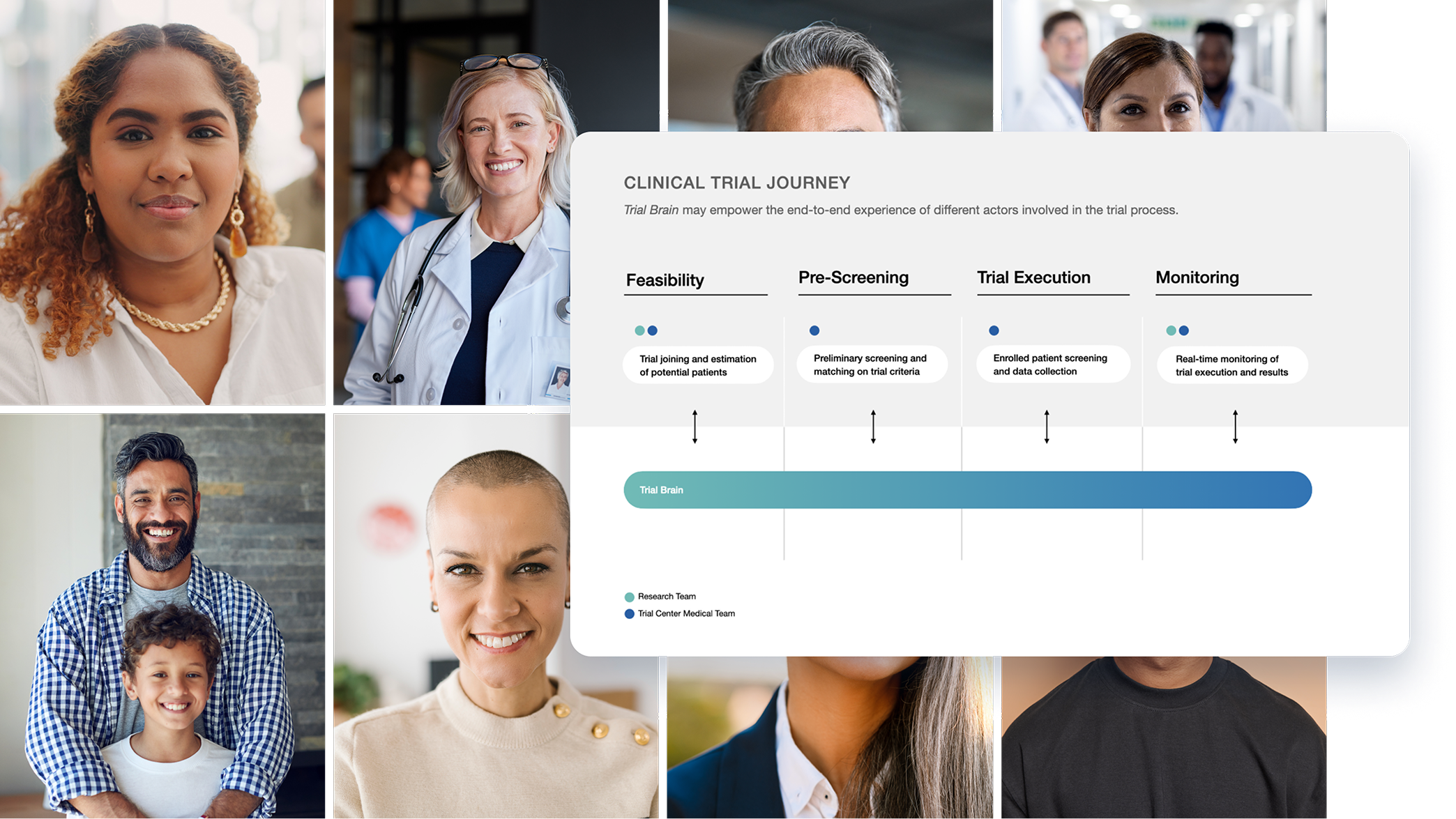
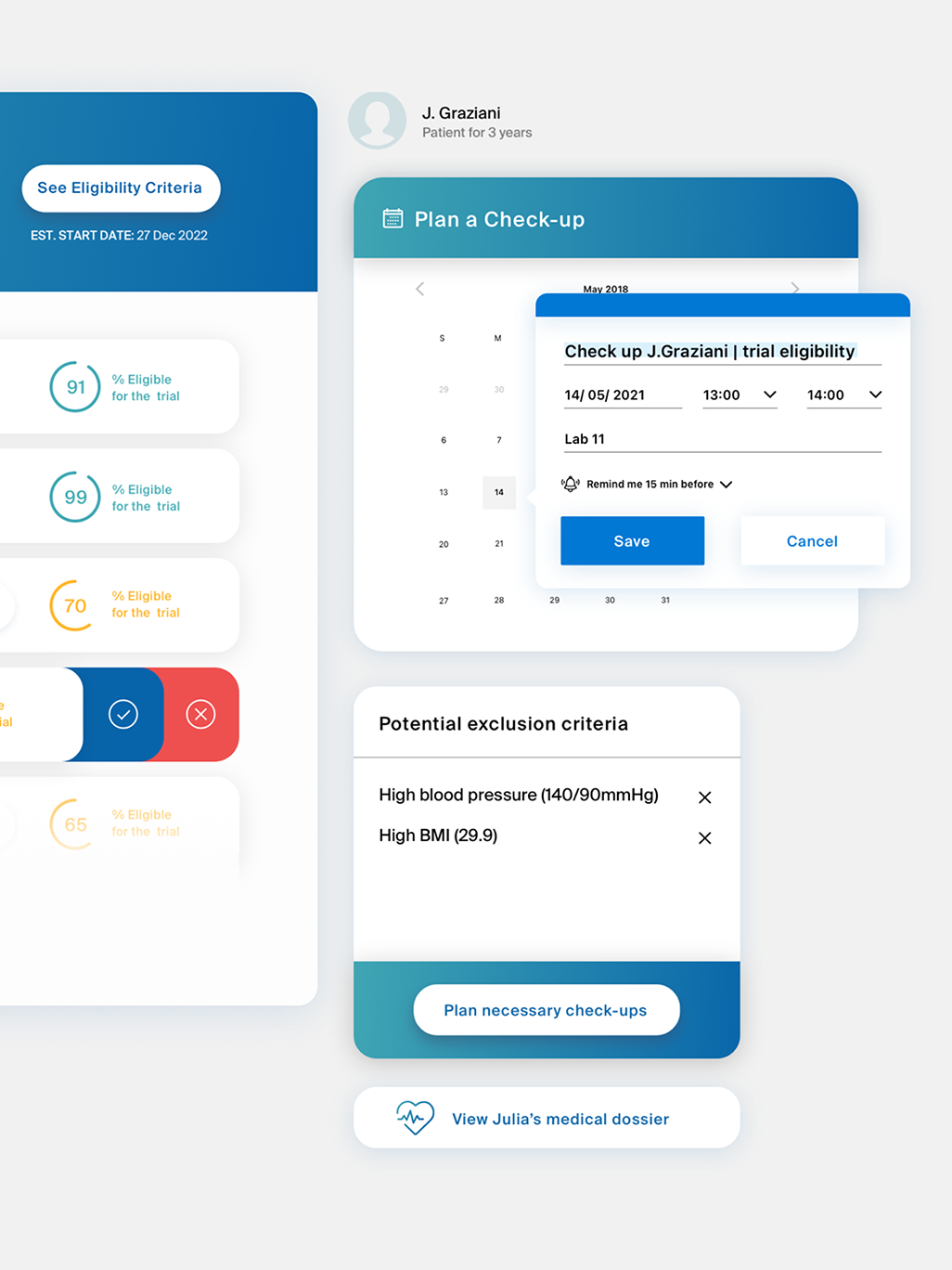
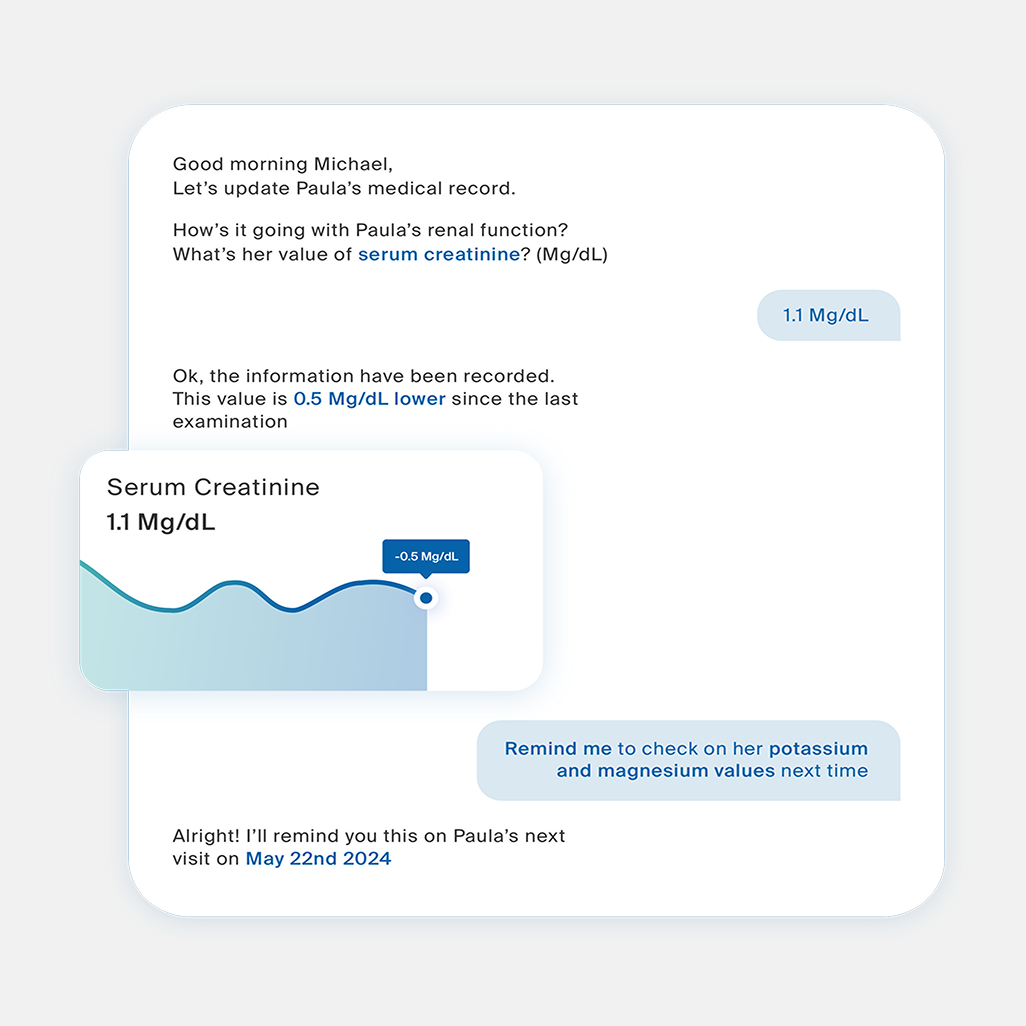
In partnership with a global big tech company, we initiated an innovative project to support tech, medical, and pharmaceutical professionals using AI. The goal was to demonstrate AI’s potential to transform clinical trial management and improve experimentation processes in compliance with the regulations and the code of ethics.